What is ionising radiation?
Radiation is energy. It can come from unstable atoms or it can be produced by machines, and travels from its source in the form of energy waves or energised particles.
But what is the difference between ionising radiation and non-ionising radiation?
Radiation that has enough energy to move atoms in a molecule around or cause them to vibrate, but not enough to remove electrons, is referred to as “non-ionising radiation”. Examples of this kind of radiation are visible light and microwaves.
Radiation that has enough energy to remove tightly bound electrons from atoms, thus creating ions, is referred to as "ionising radiation”. Examples of ionising radiation are x-rays and gamma rays.
The image below shows the forms of ionising and non-ionising radiation on the electromagnetic spectrum.
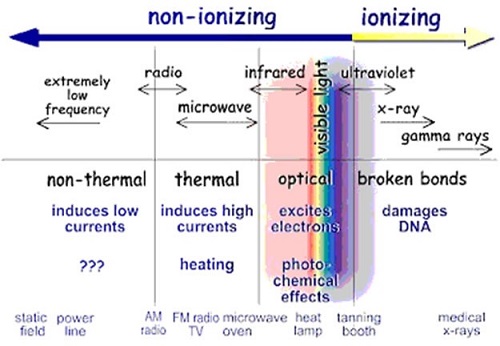
Figure 1: Types of Radiation in the Electromagnetic Spectrum, Source (EPA website)
Atoms and radiation
All matter consists of atoms. Nearly all of an atom’s mass is concentrated in the nucleus, which consists of positively charged protons and electrically neutral neutrons. Negatively charged particles called electrons orbit the nucleus. As an example, the structure of the helium atom is shown in Figure 2. Atoms have equal numbers of protons and electrons and are electrically neutral. The total number of protons and neutrons gives the mass of the atom, called the mass number.
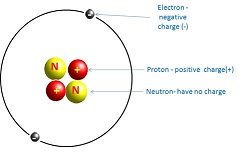
Figure 2: helium atom
Since the number of protons is unique to each element, the element together with the mass number specifies each nuclide. The nuclides of an element (i.e. atoms with the same number of protons but different numbers of neutrons) form what are called isotopes of that element. There may be several isotopes of an element. Hydrogen, for instance, has three isotopes: hydrogen-1 (common hydrogen), hydrogen-2 (deuterium) and hydrogen-3 (tritium) as illustrated in Figure 3.
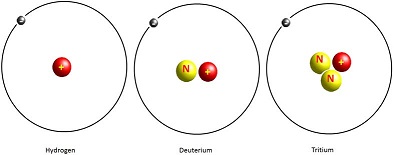
Figure 3: Hydrogen isotopes
Radioactivity and Radiation
Although many nuclides are stable, most are not. Stability is determined mainly by the balance between the number of neutrons and the number of protons a nucleus contains.
An unstable nucleus with an imbalance in the number of protons and neutrons has excess energy and will spontaneously transform into a more stable form at random by emitting radiation. Different nuclei release this energy as particles, i.e. alpha or beta particles and photons (gamma rays and X-rays).
The spontaneous transformation of nuclei is called radioactive decay. The unstable nuclide, which decays and emits radiation is called a radionuclide.
All radionuclides are uniquely identified by the type of radiation they emit, the energy of the radiation, and the half-life. The activity, used as a measure of the amount of a radionuclide present, is expressed as the number of decays per second. The activity in decays per second is often specified with the international unit the becquerel (Bq) in honour of Henri Becquerel who first identified radioactivity: one becquerel is one disintegration per second.
The activity of a specific radionuclide determines the half-life of the radionuclide. The half-life is the time required for the activity of a radionuclide to decrease to half of its initial value by radioactive decay. Half-lives for radionuclides range from tiny fractions of a second to millions of years.
Types of Radiation
Ionising radiation from radioactive materials and radiation generating equipment includes alpha and beta particles, gamma photons, X-ray photons and neutrons.
Alpha radiation consists of positively charged particles comprising two protons and two neutrons, which are emitted by radionuclides of heavy elements such as uranium, radium, radon and plutonium. Alpha radiation travels only a few centimetres in air and is blocked by a sheet of paper. Alpha radiation is not able to penetrate skin. If an alpha emitting substance is absorbed into the body, it will release all its energy to the surrounding cells. As a result, alpha-emitters can be harmful to humans if the materials are inhaled, swallowed or absorbed through open wounds.
Beta radiation consists of electrons emitted from the nucleus, is smaller than alpha particles and is able to penetrate deeper. Energetic beta radiation can penetrate human skin to the germinal layer where new skin cells are produced. It can be stopped by a sheet of metal or glass or by ordinary clothing. If beta-emitters with high energies remain on the skin for a long period of time, they may cause skin injuries such as radiation burns.
Gamma radiation is electromagnetic wave energy. Its range in air is long and its penetrating power substantial. Dense materials such as lead and concrete are good barriers against gamma rays.
X-rays are high-energy photons (like gamma radiation), which are produced artificially by the rapid slowing down of an electron beam. X-rays are similarly penetrating and, in the absence of the shielding by dense materials, can deliver significant energy to internal organs.
Neutron radiation consists of neutrons and is not in itself ionising radiation. However, if a neutron hits a nucleus, it may activate it or cause the emission of a gamma ray or charged particle thereby indirectly giving rise to ionising radiation. Neutrons are more penetrating than gamma rays and can be stopped only by a thick barrier of such as concrete, water or paraffin.
Radiation Sources
Radioactivity is a part of our earth. Ionising radiation enters our lives in a variety of ways. It arises from natural processes such as the decay of uranium and thorium in the earth, and from artificial procedures like the use of X-rays in medicine. So we can classify radiation as natural and artificial according to its origin.
Humans are exposed to natural radiation and sometimes artificial sources of ionising radiation such as medical X-rays. National and international organisations measure how much radiation the average person is exposed to per year from natural and artificial sources. Figure 4 presents a diagram of the sources and distribution of average radiation exposure to the world population published recently by the World Health Organization (WHO). As illustrated in the diagram, radon, earth gamma radiation, cosmic rays, and food and water i.e. naturally occurring radioactive materials account for 79% of the total average radiation exposure. Medical uses account for 20% whilst exposure to ionising radiation from all other man-made sources account for 1%.
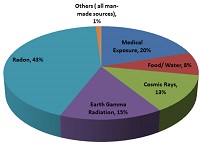
Figure 4: Sources and distribution of average radiation exposure to the world population, Source: (WHO Website)
Radiation Applications
Radiation has a wide range of applications in medical, commercial and industrial activities. In medical applications, radiation is used for imaging, measuring metabolic functions, and treatment of cancer. Industrial applications include radiography to inspect welds, pipes and other manufactured items; thickness gauges to monitor manufacturing processes; level gauges to measure flows; and analytical systems to measure compounds. Commercial applications include sterilisers to kill bacteria and pathogens; soil density gauges for highway construction; nuclear power plants for electrical power generation; and smoke detectors.
In addition, natural radioactive material may be associated with processing of mining materials including phosphate fertilisers, production and use of kaolin clays, and production and use of fossil fuels.
Radiation Effects on Health
The effect of ionising radiation on health is well studied and periodically evaluated by the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) and the International Commission on Radiological Protection (ICRP). These periodic evaluations consider how ionising radiation is measured and assess what is known about the corresponding health effects.
As a result of studies, particularly of Japanese atomic bomb survivors, it is known that exposure to radiation at moderate levels increases the risk of a person contracting cancer later in life. The risk is established to increase with the quantity of radiation absorbed (the dose), but it is very low and may even be zero at low doses. For this reason, UNSCEAR reports that it is not possible to attribute health effects in populations to long-term exposure at radiation levels typical of the global average background levels.
Although demonstrated in studies on animals, an increase in hereditary effects in human populations cannot be attributed at present to radiation exposure. One reason for this is the large fluctuation in the spontaneous incidence of these effects.
High doses of radiation such as those resulting from accidents with radiation sources, may result in radiation-induced cataracts, radiation burns and acute radiation sickness. Death will occur at very high doses.
Radiological Protection
Approaches to protection against ionising radiation are consistent throughout the world. National and international agencies regulate the activities related to ionising radiation to keep exposure to radiation ‘as low as reasonably achievable’, a concept termed ‘ALARA’. There are three principles of radiation protection recommended by the ICRP namely justification, optimisation, and dose limitation.
Justification requires that any proposed activity that may cause exposure to people should yield a sufficient benefit to society and individuals to justify the risks incurred by the radiation exposure.
Optimisation, related to the practice of ALARA, requires that the radiation exposures resulting from the practice must be reduced to the lowest level possible considering the cost of such a reduction in exposure or dose.
While dose limitation involves setting upper limits on the dose that may be received by any individual member of the public or worker from exposures (other than medical exposures).